The True ROI Of Decentralized Clinical Trials
Home » The True ROI Of Decentralized Clinical Trials
Financial return on investment is the language of pharma’s C-suite – this astute observation was made by Ken Getz, MBA (Executive Director of the Tufts Center for the Study of Drug Development) during his keynote talk at DPHARM 2024.1 The unspoken element behind this, it that it does not matter how big, or how small the pharma biotech is – the finances need to make sense for it to be possible to conduct potentially life-saving research.
It is with this in mind that we need to consider how solutions are presented to leadership teams – when they are responsible for the fiscal bottom line, this perhaps that needs to be the first concern addressed. How can DCTs increase quantitative trial ROI?
Defining DCTs
Decentralized Clinical trials, often abbreviated to DCTs, have had their definition redefined several times over, either amalgamated to fit a specific narrative or adjusted to try and broaden understanding. In the FDA’s most recent guidance, there has been a notable switch from using the term “Decentralized Clinical Trials”, to instead the terminology “Decentralized Trial Elements.”
There is clarity in comprehension, and so it is important that we review our definition of what a “decentralized clinical trial” is in the context of this discussion. This definition will be the bedrock on which we measure return on investment, as well as how to best implement DCTs for maximum return.
For the purpose of this article, we will consider the following three references to Decentralized Clinical Trials and their definitions and relationship to each other:
Traditional Trials: Trials that are run specifically in urban-based academic or private research centers.2
Decentralized Clinical Trials: An umbrella term for trials that make use of DCT elements -hybrid, remote, or virtual trials – to enable them to operate beyond traditional trial site settings.3
Decentralized Trial Elements: The broad range of tools, services and solutions that are leveraged in order to bridge accessibility gaps faced by sponsors, sites, and participants.3
Why DCTs At All?
Given that we live in the post pandemic world, the question really has to be why not DCTs? Every other aspect of life now has a “remote” or “hybrid” option – work, shopping, education, and even general health check-ups. The result is that adults across all generations now expect to have these options available to them, so why would they anticipate anything different when it comes to clinical trial participation.4,5
And this expectation could be considered a fair one – especially since it was so successfully utilized during the Covid-19 pandemic.6 Not only was there great publicity around the fact that clinical trials could be conducted in remote settings, but the results were tangible the world-over. While incredibly impacted, the industry still found a way to deliver results through decentralized methods and is expected to continue to do so. And why not – we are, after all, working in an industry that is known for constantly pushing the boundaries with cutting-edge research and innovation, in an effort to bring the results of that to market faster.
On-paper, there is no reason why DCTs should not yet have had wide-spread adoption. With the global pandemic being proving ground for success, DCTs have shown that they have both the popularity with participants and long-term cost efficiencies to go the distance. So why haven’t they?
Potential Blocker: Risk Aversion
While this is not something that you would expect from an industry that constantly pushes the realms of possibility, it makes sense during times of economic instability. Despite the potential ROI from DCTs, when the amount of funding pharmaceutical/biotech companies may have available is limited or restricted, their willingness to adopt new solutions and veer away from the path of “tried and tested” rapidly declines, despite these tried and tested methods no longer yielding the desired results.7
With the majority of sponsors and clinical teams having little, to no experience with DCT elements, nor the understanding of how to implement and support the end-users (sites and participants) of these solutions, it makes sense that there has been resistance to move away from what they know, as the markets have not been kind to the pharmaceutical / biotech industry since the end of the pandemic. Both economic and political factors have influenced a down-turn in sentiment – approximately a drop of 15.2% in the Association of Investment Companies’ Biotechnology & Healthcare sector over five years (September 2020 – September 2025).8
Potential Blocker: Perceived Financial Constraints
Hand-in-hand with risk aversion, is the financial limitations that many pharmaceutical/biotech companies are faced with, irrespective of size. Every spend needs to be accounted for, and no one will agree to fund a concept they do not believe will provide some form of ROI.
While some still perceive DCTs as high-cost initiatives with uncertain returns, growing evidence shows that when implemented strategically, DCTs can deliver significant ROI. However, these benefits rely heavily on choosing the “right” service provider – selecting one that will consider an approach that isn’t “off the shelf” but will truly consider the trial and understand objectives, offering a solution that keeps return on investment at the forefront of solution design – early in the trial planning process.
Doing this from the outset enables them to work with the study design team and provide input that aligns with study objectives. This approach is critical in order to avoid costly last-minute fixes that often rapidly drive-up expenses and reduce efficiency.
Further, engaging in transparent, well-informed service provider discussions early on ensures that DCT approaches are not only cost-effective but also capable of producing both measurable outcomes and meaningful participant experiences.
Potential Blocker: AI, DCTs And The Need For Regulators To Catch-Up
While DCTs have been working on their breakthrough into the mainstream, AI developments have now reached the stage where, when implemented alongside DCT solutions, they can supercharge ROI and accelerate trial timelines into new stratospheres.9
For those tools, such as AI, that are still in their relative infancy, that may seem like the best approach, however, fortune favors the brave and those that push for early adoption will likely see themselves outpacing their competitors. The other knock-on effect is that DCT solutions that have been approved and effectively implemented, such as in-home nursing services like Home Trial Support, have been incorrectly categorized as ‘new and untested’10 even though it has been successfully recruiting and retaining participants in trials for almost 20 years.
Finding The Solutions
The blockers to potential uptake of DCT solutions are valid but should not be used as reasons not to move forward. Rather, they should be used as guides to help sponsors find the right balance between financial costs and innovation.
A key aspect to this lies in building a trial that balances the needs of all those involved while still delivering on results.
Solution: A Customized Approach
While traditional trial methods follow a standardized approach, DCTs offer flexibility – there is no one size fits all when it comes to DCT methods. What may work for one trial protocol may not work for another – even if they are in the same therapeutic indication and designed to treat the same facet of the disease. They will, undoubtedly, have separate needs in order for them to succeed. This means that the approach to applying decentralized trial elements to protocol design will need to be considered ONLY in relation to that trial.
This does not mean we cannot examine similar trials in similar indications to observe what has and has not worked in the past, but rather avoid using DCT solutions as templates that have a blanket application. By working with a service provider, it is possible to determine which DCT element will enable true optimization. Key considerations that should be undertaken when selecting which DCT elements to use must include the needs and capabilities of both sites and participants, as well as regulatory and ethical requirements and restrictions:
Participant Considerations, examples:
- Location: Urban vs rural
- Connectivity: Internet reliability and limitations,
- Language: Communication barriers including technology literacy
- Cultural: Customs, etiquette, socio-economic circumstances, and more
Site Abilities, examples:
- Experience: Investigator & study team capacity and research experience
- Infrastructure: Site facilities, equipment and logistics
- Location: Geographic location of the site
- Access to participants: Population pool and ability to recruit
Regulatory Requirements, examples:
- Technology: Use of telemedicine, wearables, remote informed consent etc.
- Data Protection: privacy and security considerations
- Remote Procedures: Clinical tests and procedures, if any, that can be done outside of the home
- Training: Requirements for in-home healthcare professionals (HCPs), site staff and/or participants
These needs must be reviewed, otherwise the use of DCT elements can and will be more costly in the long term. By not weighing up the implications for participants and sites, while also factoring in regulatory requirements, sponsors face the risk of over-burdening sites, harming data quality, slowing participant recruitment and retention, and inhibiting trial activities due to regulatory and ethics adjustments.
By working with the right service provider, sponsors will also be able to evaluate and consider their needs and limitations:
- Suitability of the investigational product (IP)
- Logistical implications of DCT implementation
- Quality, risk management, and participant safety
- Trial design & protocol complexity
- Budget
Service providers should work with sponsors to design and deliver the right DCT elements for each specific trial. By taking a nuanced approach to the trial design, it can be fully optimized to yield the best results.
Solution: Measuring Metrics For Quantitative ROI
As it is with any investment, there are several upfront costs that may be incurred. These are often financial and logistical when it comes to DCT implementation – the extent of which is determined by the chosen approach.
Key to tracking quantitative success lies in measuring the success of the DCT elements that have been employed. These may have been multiple DCT elements working together to provide an integrated solution or fully standalone alone solution – dependent on their set goals and objectives. Further, to fully appreciate the ROI of the employed DCT strategy, a mathematical framework must be utilized to measure success in “trade-offs.” For example, there may be a higher cost to utilizing an in-home HCP to conduct some of the study visits, however, the trade-off comes in the form of reduced participant attrition and increased enrollment and retention – which ultimately prevent trial delays and even accelerates completion dates.7
Taking it even further, the Tufts Center for the Study of Drug Development (Tufts CSDD) leveraged the industry-standard expected net present value (eNPV) methodology (often used to assess the financial viability of a project by accounting for R&D cash flows, market access risks, commercialization costs, and other uncertainties) in order to provide evidence supporting the quantitative benefits that trials using DCT elements can deliver.11
The results from the Tufts CSDD study showed clearly measurable results, with reductions in phase cycle times – a “trade off” from DCT utilization – being a key contributor to ROI. Based on a benchmark dataset of 160 phase 2 and phase 3 clinical trials, the results found that trials deploying one or more DCT element resulted in:
- (near) 5x ROI for Phase 2 trials11
- 13x ROI for Phase 3 trials11
Just as the participation of each trial participant contributes to the success of a clinical trial study, so does each DCT element used in a trial. However, individual DCT elements have not always been tracked, and therefore not included in failure or success attributions.
It can also be a way to measure patient-centricity. For example: MRN worked with a global pharmaceutical company, evaluating Home Trial Support (HTS) with direct-to-patient investigational product shipments to support rare disease participant enrollment and retention. Balancing the cost of these two DCT elements with the cost savings determined by achieving the study completion date, these solutions kept trial costs approximately net neutral. Considering that an average of 25.5% of participants drop out of trials, and 90% of studies are delayed due to low retention,12 it becomes more important than ever to measure the successes of effectively deployed DCT elements.
To ensure accurate, quantitative (and qualitative) ROI on DCT implementation it is critical to define the expected outcomes the DCT elements employed will achieve.
Solution: Regulatory Experience & Expertise For Acceleration
Time is arguably the most expensive resource in clinical trials – and unnecessary delays can become very costly, very quickly. Adding a DCT element can make regulatory approvals more complicated, especially for global studies. Yet, these complications can be managed, it just takes planning and partnership – simple, if you know how.
This is where experienced providers play a pivotal role. Identifying a knowledgeable, trusted service provider or service provider right at the start of the trial’s planning phase, will enable them to work with the trial design team to not only identify the right DCT elements required to optimize their solution ROI, but also on how to integrate and execute on them to ensure regulatory requirements expectations are met.
Working with the right service provider becomes even more crucial when it comes to multi-arm and/or global studies, as the regulatory requirements and submission processes can greatly vary – leading to unwanted and, often, avoidable delays.
Crunching The Numbers – Success With HTS
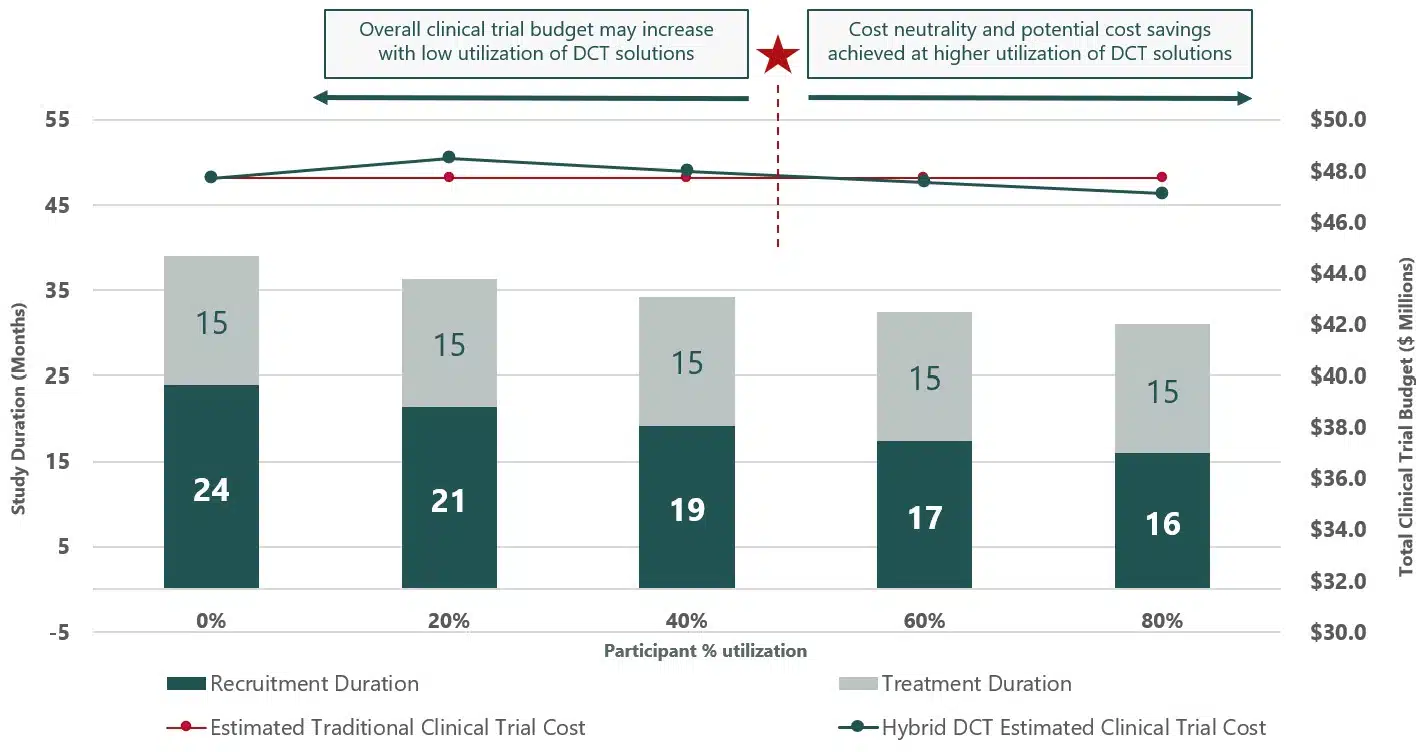
MRN used a case study where there was 20% participant adoption of the DCT solution, in-home trial visits conducted by trained healthcare professionals (HTS), along with internal data on clinical trial costs to create a baseline to measure overall financial performance with or with the incorporation of DCT elements (see fig. 1). This was then extrapolated to measure the potential success when there was greater integration of the HTS DCT solution.
The results of this showed that the more participants that opted to utilize HTS, trial costs decreased due to timeline decreases and trials completed faster. When there was 80% participant uptake of HTS, trial duration shrunk by 8 months, greatly reducing overall trial costs.
Fig 1.
Conclusion
As Ken Getz rightly observed, ROI is the language of the C-suite – and it’s the one that ultimately determines whether a clinical trial gets off the ground or not. Regardless of the size of the organization, the finances have to be sound, and when it comes to decentralized clinical trials, ROI needs to become the lens through which they are implemented and measured.
MRN’s data around Home Trial Support makes a compelling case for what DCT elements can deliver when implemented early and at scale. Starting with just 20% adoption, the financial model already showed encouraging results. But when that figure rose to 80% participant uptake, the numbers became impossible to ignore—trials shortened by up to eight months, with overall costs falling due to faster completions and fewer delays.
DCTs are a proven, adaptable model that, when implemented strategically and early, can enhance trial performance, improve participant engagement, and deliver measurable financial and operational returns. The barriers that remain, perceived cost, regulatory ambiguity, and risk aversion, are not insurmountable. Rather, they highlight the importance of planning, customization, and strategic partnership.
Crucially, DCTs must not be approached with a cookie-cutter mindset. Every protocol brings unique requirements that demand a tailored approach. By aligning trial design with participant realities, site capabilities, and evolving regulatory frameworks, sponsors can mitigate risk and maximize return. Yes, we’re navigating a complex environment, but complexity isn’t a reason to stall. It’s a reason to plan better.
Ultimately, DCTs don’t need to be sold as “the future” – they are already here. And when implemented strategically, and their metrics are measured correctly, they offer tangible returns: time saved, costs reduced, participant access improved, trials completed, new therapies to market.
It’s not about reinventing the wheel. It’s about building the right version of it for your trial—measurable, flexible, and built to deliver results.
References:
- Getz K. ROI on DCTs in Clinical Trials, from Tufts Center for Study of Drug Development. DPharm 2024 Keynote Presentation, 20 Feb 2024. https://theconferenceforum.org/webinars/roi-on-dcts-in-clinical-trials-from-tufts-center-for-study-of-drug-development. Last Accessed October 2025.
- Goodson N, et al. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. npj Digit. Med. 5, 58 (2022). https://doi.org/10.1038/s41746-022-00603-y. Last Accessed October 2025.
- U.S. Food and Drug Administration. Conducting Clinical Trials With Decentralized Elements. September 2024.
- YouGov. Global consumers embrace remote activities post-pandemic. 9 June 2023. https://yougov.com/articles/46777-global-consumers-embrace-remote-activities-post-pandemic. Last Accessed October 2025.
- McKinsey & Company. Americans are embracing flexible work—and they want more of it. 23 June 2022. https://www.mckinsey.com/industries/real-estate/our-insights/americans-are-embracing-flexible-work-and-they-want-more-of-it. Last Accessed October 2025.
- Manjrekar K, et al. eP187: Decentralization of clinical trials in the era of COVID-19: Implications for rare disease trials. Genetics in Medicine, Vol24:3,March 2022.
- Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. 2018 Aug 7;11:156-164.
- The Association of Investment Companies. ‘Sentiment is as poor as we’ve seen’: What now for healthcare trusts?. 11 September 2025. https://www.theaic.co.uk/aic/news/industry-news/sentiment-is-as-poor-as-weve-seen-what-now-for-healthcare-trusts. Last Accessed October 2025.
- McKinsey & Company. Faster, smarter trials: Modernizing biopharma’s R&D IT applications. 13 February 2025. https://www.mckinsey.com/industries/life-sciences/our-insights/faster-smarter-trials-modernizing-biopharmas-r-and-d-it-applications. Last Accessed October 2025.
- Askin S, et al. Artificial Intelligence Applied to clinical trials: opportunities and challenges. Health Technol (Berl). 2023;13(2):203-213.
- DiMasi JA, et al. Assessing the Financial Value of Decentralized Clinical Trials. Ther Innov Regul Sci 57, 209–219 (2023).
- Poongothai S, et al. Strategies for participant retention in long term clinical trials: A participant -centric approaches. Perspect Clin Res. 2023 Jan-Mar;14(1):3-9.